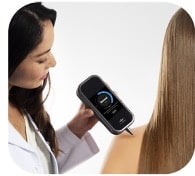
The DermaSensor device is an affordable, handheld device that uses machine learning and spectroscopy to automatically test skin lesions for potential cancer.
z
Miami-Based DermaSensor, Inc. Raises Additional $10 Million To Prepare for U.S. Launch
MIAMI, FLORIDA, UNITED STATES, June 15, 2022 /EINPresswire.com/ — DermaSensor Inc., a health technology company designing non-invasive tools to better equip primary care physicians (PCPs) to detect skin cancer, has successfully met all primary endpoints for their two FDA pivotal studies. To fund U.S. commercial launch preparations the company has raised an additional $10 million. Both of these announcements come as they prepare for an expected U.S. launch, which would make DermaSensor the only FDA-cleared, automated skin cancer detection tool on the market that uses any kind of imaging or optical technology.
The DermaSensor device was awarded Breakthrough Device Designation by the FDA in 2021. The company expects this to be the first device cleared by the FDA to assist PCPs’ in evaluating skin cancer. The device uses a form of optical spectroscopy called Elastic Scattering Spectroscopy (ESS) to take non-invasive samples of tissue, capturing cellular-level information from the skin lesion using hundreds of wavelengths of light, similar to how sonar uses sound. The DermaSensor platform provides output directly to the user, without the need for a laboratory or another physician to analyze the spectral data, as the proprietary algorithm immediately assesses the data and provides a result in seconds.
“Published studies have already shown the potential for ESS to identify cancer using large, counter-top spectroscopy systems,” says Dr. Leffell, Chief of Dermatologic Surgery and skin cancer researcher at the Yale School of Medicine and a medical advisor to DermaSensor. “Miniaturizing that technology into a hand-held, point-and-click tool significantly improves its accessibility and utility for time-constrained physicians, who commonly must make quick referral decisions for skin lesions. And, most importantly, we hope the use of our tool will help clinicians identify skin cancer sooner, since 99% of skin cancers are curable if detected early.”
$10 Million in New Capital Will Fuel U.S. Launch Preparations
The close of another oversubscribed financing for the company, led by existing investors including Ceros Capital Markets, comes as they begin preparations for the expected U.S. commercial launch following FDA clearance. Accordingly, the company is hiring for a range of roles, including Chief Commercial Officer. “This $10 million in additional capital is a clear vote of confidence, from both existing and new investors, in our strong clinical evidence and in the potential of our product to greatly improve the detection of skin cancer by PCPs. This could provide major public health benefits since skin cancer is the most common cancer and there are more primary care physicians than any other specialty,” says Cody Simmons, the CEO of DermaSensor Inc.
The company’s FDA pivotal clinical study, DERM-SUCCESS, was successful in meeting both of its primary endpoints. DERM-SUCCESS is a prospective, blinded study with over 1,000 patients and conducted by 22 primary care study centers globally, including Mayo Clinic. The company also successfully met both primary endpoints for its second pivotal study, which involved over 100 physicians and showed that their use of the DermaSensor test results improved their detection of skin cancer. The company believes these studies mark the first ever successful FDA pivotal studies for any type of skin cancer device for PCPs.
In addition to these two FDA pivotal studies, the company also recently published another prospective study titled DERM-ASSESS III, conducted by 10 dermatology study centers globally, including Dana-Farber Brigham Cancer Center. The study enrolled over 500 lesions suspicious for melanoma and demonstrated the product’s effectiveness at detecting melanoma while ruling out benign lesions. The results from this study were published as a late-breaking presentation at the American Academy of Dermatology conference in Boston in March 2022. “In addition to five other previously completed clinical studies, we have also now successfully completed three of the largest prospective clinical validation and clinical utility studies ever done for regulatory clearance for any kind of skin cancer detection tool,” says Simmons, “And having already complied with regulatory requirements in Europe, Australia and New Zealand, we look forward to working with the FDA through the premarket review process.”
DermaSensor Inc. is a health technology company designing non-invasive tools to better equip primary care providers for skin cancer detection. The DermaSensor device is an affordable, handheld tool that uses machine learning and spectroscopy to evaluate skin lesions for potential cancer in a matter of seconds. DermaSensor’s mission is to improve outcomes and save on healthcare costs by providing broad access to effective skin cancer checks since most Americans do not receive an annual skin exam. The DermaSensor device is currently CE Marked and it is registered and available for sale in Australia and New Zealand. It is currently an experimental device limited to investigational use in the United States and is not available for U.S. sale.
Marc Acton
Alpha Echo Agency, LLC
+1 615-267-8450
email us here
Visit us on social media:
LinkedIn

