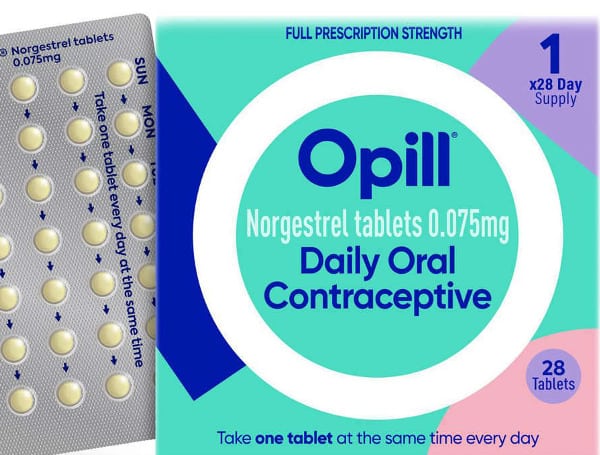
On Thursday, the Food and Drug Administration (FDA) approved the oral contraceptive Opill for over-the-counter sales, making it the first hormonal contraceptive pill available in the U.S. without a prescription.
“Approval of this progestin-only oral contraceptive pill provides an option for consumers to purchase oral contraceptive medicine without a prescription at drug stores, convenience stores and grocery stores, as well as online,” according to the FDA.
The timeline for availability and price of this nonprescription product is determined by the manufacturer. Other approved formulations and dosages of other oral contraceptives will remain available by prescription only.
In the news: Florida Abortion Clinic, State Reach Settlement
“Today’s approval marks the first time a nonprescription daily oral contraceptive will be an available option for millions of people in the United States,” said Patrizia Cavazzoni, M.D., director of the FDA’s Center for Drug Evaluation and Research.
Opill, also known as the “mini-pill,” contains one hormone, progestin, and is taken daily. It was first approved by the FDA as a prescription in 1973.
The FDA said it is approving the over-the-counter version for all users of reproductive age, including teenagers, a move that is expected to remove barriers to access and reduce the risk of unintended pregnancies.
“When used as directed, daily oral contraception is safe and is expected to be more effective than currently available nonprescription contraceptive methods in preventing unintended pregnancy,” said Cavazzoni.
The most common side effects of Opill include irregular bleeding, headaches, dizziness, nausea, increased appetite, abdominal pain, cramps or bloating.
In the news: GOP Presidential Candidates Split On Nationwide 15-Week Abortion Ban
Opill should not be used by those who have or have ever had breast cancer. Consumers who have any other form of cancer should ask a doctor before use. Opill also should not be used together with another hormonal birth control product such as another oral contraceptive tablet, a vaginal ring, a contraceptive patch, a contraceptive implant, a contraceptive injection or an IUD (intra-uterine device).
Android Users, Click To Download The Free Press App And Never Miss A Story. Follow Us On Facebook and Twitter—signup for our free newsletter.
We can’t do this without your help; visit our GiveSendGo page and donate any dollar amount; every penny helps